 |
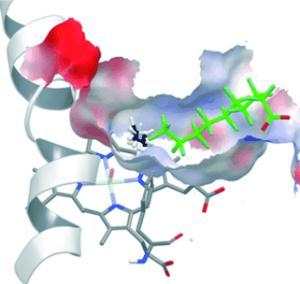
A new spin - The addition of chemically inert perfluoro carboxylic acids (green; see picture) to P450 enzymes results in dramatic activation of their catalytic activity as a result of the conversion of the Fe/heme from a low-spin to a high-spin state, and the reduction of the binding-pocket size. Together these effects allow otherwise inert substrates such as propane and even methane to be oxidized.
[Credit: Angewandte Chemie International Edition] |
Methanol is a useful starting material for many chemical syntheses, and it can also be added to conventional fuels to drive fuel cells. Conventional processes for producing methanol from methane involve detours (synthesis gas), are markedly complex and energy intensive, and require high temperatures and pressures. Nature, on the other hand, has a much more elegant route: the enzyme methane monooxygenase does the job gently and efficiently. Unfortunately this is a very complex enzyme that cannot easily be produced and used in an artificial environment. The cytochrome P450 (CYP) family of enzymes could represent an alternative starting point. The main job of these enzymes is the oxidation of various substances produced by the body or introduced to it. In the reaction, carbon–hydrogen bonds are oxidized to make alcohol groups (–OH). The active component of these enzymes is a heme, an iron–porphyrin complex similar to that in our hemoglobin.
The problem is that the binding pocket of this enzyme is just too big to snugly bind and oxidize small molecules such as methane. Instead of trying to devise complex methods to create a suitable enzyme, Reetz and his co-workers came up with a clever trick: chemically “tuning” a CYP enzyme. The scientists added an additional guest into the binding pocket in order to make it smaller.
The natural substrates for CYP enzymes are fatty acids. As a guest molecule, the researchers chose a compound that resembles a fatty acid, a carbonic acid in which all of the hydrogen atoms in the hydrocarbon chain have been replaced with fluorine atoms. This type of molecule is as water-repellent as the original, but takes up more room. The fluorine atoms make it chemically inert so that it does not participate in any reactions. Like the molecule it is modeled on, this guest is able to bring the iron-heme complex of the enzyme into its catalytically active state (high-spin state). The significantly smaller binding pocket now allows methane to bind effectively so that it can be oxidized to methanol.
Says Reetz: “The road to success is still far for a technical implementation, yet, the concept opens up new perspectives for the development of further reactions, such as the oxidation of other chemical compounds.”



