 |
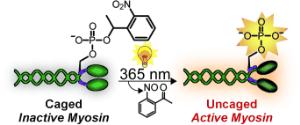
Shining light on myosin: The incorporation of a caging group onto the essential phosphoserine residue of myosin by protein semisynthesis enables light-triggered activation of the protein (see picture). Caging eliminates the myosin activity, but exposure to 365 nm light restores its function to native levels. The caged protein can also be introduced into cells to facilitate studies of myosin with precise spatial and temporal resolution.
[Credit: Angewandte Chemie International Edition] |
In order for our muscles to contract, two types of fibrous proteins, myosin and actin, must interact. Driven by splitting of the cellular fuel adenosine triphosphate (ATP), “buttons” on the myosin molecules attach, allowing the myosin to dangle off of the actin filaments. In non-muscular cells, myosin ensures that the cell constricts itself in the division process. Myosin consists of several different protein chains. The activity of non-muscular myosin is regulated through its regulatory light chain. As soon as a phosphate group binds to a specific site (Ser19) of the light chain (phosphorylation), myosin become active. The activity can be amplified through binding of a second phosphate group at a neighboring site (Thr18).
Myosin has been intensively studied. However, it has not been possible to examine precisely what happens after activation of the molecule in living cells both spatially and over time. This research team has now found a trick that makes real-time observations possible: A myosin molecule that can be switched on by light. To achieve this, the researchers used protein synthesis to produce a synthetic regulatory chain that already contains one or two phosphate groups. The trick is that one of the phosphate groups is covered by a cage. In this form, the chain is inactive. Irradiation with light makes the cage split off, switching on the regulatory chain and activating the myosin.
The researchers replaced the natural light chain in myosin molecules with their synthetic one and introduced this light-activated myosin into cells. Irradiation activates it at a defined time in a defined place. In this way, the researchers hope to observe what happens after the activation of myosin in a cell in real time.



