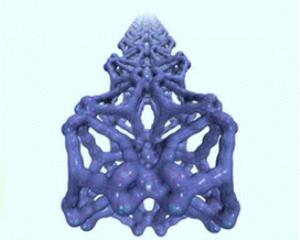
Named NU-109 and NU-110, the materials belong to a class of crystalline nanostructure known as metal-organic frameworks (MOFs) that are promising vessels for natural gas storage for vehicles, catalysts, and other sustainable materials chemistry.
The materials' promise lies in their vast internal surface area. If the internal surface area of one NU-110 crystal the size of a grain of salt could be unfolded, the surface area would cover a desktop. Put another way, the internal surface area of one gram of NU-110 would cover one-and-a-half football fields.
A paper describing the findings, 'Metal-organic Framework Materials with Ultrahigh Surface Areas: Is the Sky the Limit?' was published August 20, 2012 in the Journal of the American Chemical Society [see below].
The research team, led by Omar Farha, research associate professor of chemistry in the Weinberg College of Arts and Sciences, has synthesized, characterized, and computationally simulated the behavior of the two MOFs that display the highest experimental Brunauer-Emmett-Teller surface areas of any porous material on record, 7,000 m2/g; that is, one kilogram of the material contains an internal surface area that could cover seven square kilometers. (Brunauer-Emmett-Teller, or BET, is an analysis technique for measuring the surface area of a material.)
The extremely high surface area, which is normally not accessible due to solvent molecules that stay trapped within the pores, was achieved using a carbon dioxide activation technique. As opposed to heating, which can remove the solvent but also damage the MOF material, the carbon dioxide-based technique removes the solvent gently and leaves the pores completely intact.
The development could rapidly lead to further advances. MOFs are composed of organic linkers held together by metal atoms, resulting in a molecular cage-like structure. The researchers believe they may be able to more than double the surface area of the materials by using less bulky linker units in the materials' design.
The research comes from the labs of Joseph T. Hupp, professor of chemistry in Weinberg, and Randall Q. Snurr, professor of chemical and biological engineering at the McCormick School of Engineering.
Other authors include SonBinh Nguyen, professor of chemistry in Weinberg; Ibrahim Eryazici, Nak Cheon Jeong, Brad G. Hauser, Amy A. Sarjeant, and Christopher E. Wilmer, all of Northwestern; and A. Özgür Yazaydin of the University of Surrey in the United Kingdom.
The MOF-designing and -synthesizing technology is being commercialized by NuMat Technologies, a Northwestern startup that has won more than $1 million in business plan competitions since incorporating in February.
Further Information:
Omar K. Farha, Ibrahim Eryazici, Nak Cheon Jeong, Brad G. Hauser, Christopher E. Wilmer, Amy A. Sarjeant, Randall Q. Snurr, SonBinh T. Nguyen, A. Özgür Yazaydin, Joseph T. Hupp:
Metal–Organic Framework Materials with Ultrahigh Surface Areas: Is the Sky the Limit?.
In: Journal of the American Chemical Society; J. Am. Chem. Soc., published online 20 August 2012, DOI 10.1021/ja3055639
Source: Northwestern University, USA
Last update: 07.09.2012
Perma link: https://www.internetchemistry.com/news/2012/sep12/ultrahigh-surface-areas.php
More chemistry: index | chemicals | lab equipment | job vacancies | sitemap
Internetchemistry: home | about | contact | imprint | privacy
© 1996 - 2023 Internetchemistry
