African sleeping sickness is caused by the tropical parasite Trypanosoma brucei. This unicellular, worm-shaped organism is endemic in Sub-Saharan Africa.
It can be transmitted to humans through the bite of the tsetse fly. Infected people first get headaches and aching limbs, after which confusion, spasms and other symptoms follow at a later stage. Finally, the patients go into some kind of vegetative state and die.
About 30,000 new infections occur each year.
There are still no vaccines available against the pathogen and the drugs on offer have in part extreme side effects.
Therefore, better drugs are urgently needed for the treatment of this disease.
This is what pharmacologists, medical researchers and biologists of the University of Würzburg are currently working on. Successfully too: The scientists present a new potent agent against trypanosoma in the Journal of Medicinal Chemistry.
Quinolone amide disrupts the cell division of the parasites
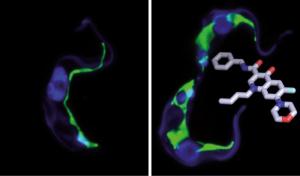
The new agent is a molecule classified into the group of quinolone amides. It has been developed by Würzburg pharmacologists; in cell cultures, the agent reliably kills off the pathogens responsible for the sleeping sickness - even when administered in small doses.
How does the quinolone amide attack the parasites? Analyses conducted at the Biocenter have shown that the agent interacts with the so-called kinetoplasts. These structures are only found in trypanosomes. "Without the kinetoplasts, the cell division and consequently the reproduction of the pathogens halts," says Nicola Jones at the Biocenter.
Objective: To make the agent soluble in water
Next, it must be clarified in an animal model whether the new agent works effectively in an infected organism as well. However, there is still a hurdle to be cleared before doing this. Quinolone amide is poorly soluble in water. "Therefore, processing it into a drug is very difficult; furthermore, the agent is not absorbed into the blood effectively enough," explains Georg Hiltensperger at the Department of Pharmaceutical Chemistry.
So the bioavailability of the agent still needs to be improved. In order to achieve this, the researchers pursue two strategies. Firstly, they are testing whether the quinolone amide can be made better soluble in water without loss of effectiveness by means of chemical modifications. Secondly, they are trying to encapsulate the agent with pharmaceutical technologies so effectively that it is delivered to the blood in sufficient quantities after oral administration.
Groups involved in the research
The scientists at the University of Würzburg involved in this research include the study groups of professors Ulrike Holzgrabe and Lorenz Meinel (Pharmacy), Markus Engstler (Biology) and Holger Braunschweig (Chemistry). The tropical medicine expert August Stich of the Medical Mission Institute Würzburg and the Basel researcher Marcel Kaiser are also contributing to this research.
The results were achieved at the Collaborative Research Center (SFB) 630 (Recognition, Preparation and Functional Analysis of Agents against Infectious Diseases) of the University of Würzburg. The SFB is funded by the German Research Foundation.
Further Information:
Georg Hiltensperger, Nicola G. Jones, Sabine Niedermeier, August Stich, Marcel Kaiser, Jamin Jung, Sebastian Puhl, Alexander Damme, Holger Braunschweig, Lorenz Meinel, Markus Engstler, Ulrike Holzgrabe:
Synthesis and Structure-Activity Relationships of New Quinolone-Type Molecules against Trypanosoma brucei.
In: Journal of Medicinal Chemistry; J. Med. Chem., 2012, 55 (6), pp 2538 - 2548, DOI 10.1021/jm101439s
Source: Julius-Maximilians University, Würzburg, Germany
Last update: 15.07.2012
Perma link: https://www.internetchemistry.com/news/2012/jul12/quinolone-amide-parasites.php
More chemistry: index | chemicals | lab equipment | job vacancies | sitemap
Internetchemistry: home | about | contact | imprint | privacy
© 1996 - 2023 Internetchemistry
