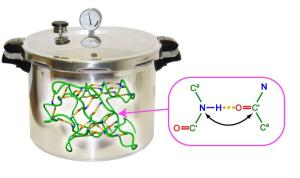
Proteins usually carry out their biological function when their polypeptide chain is arranged into a stable three-dimensional structure.
The specific structure of a protein is stabilized by numerous hydrogen bonds that connect individual amino acids.
Using an innovative method, namely Nuclear Magnetic Resonance Spectroscopy (NMR Spectroscopy) in combination with high pressure, Dr. Nisius and Prof. Grzesiek from the Biozentrum of the University of Basel have provided important new insights into the hydrogen bond network of Ubiquitin and its importance for the stability of this model protein.
Their findings have now been published in the renowned scientific journal Nature Chemistry [see below].
Proteins consist of a sequence of amino acids and have important physiological functions, such as catalysis or transport of metabolic products. To perform their physiological role, proteins need to fold their linear amino acid chains into a stable three-dimensional structure. In part, the spatial arrangement is determined by a network of hydrogen bonds. However so far it was unclear to what extent individual hydrogen bonds contribute to the stability of a structure. Using a newly developed high pressure cell and NMR method Dr. Nisius and Prof. Grzesiek could, for the first time, completely characterize the stability of individual hydrogen bonds in the protein Ubiquitin.
Particular stability of key, long-range hydrogen bonds
The stability of a thermodynamic system, such as a protein, can be analyzed by subjecting it to variations in pressure and temperature. Using high resolution NMR methods and a newly developed pressure cell Nisius and Grzesiek have precisely analyzed the contributions of 31 backbone hydrogen bonds to the conformational stability of the model protein Ubiquitin. The pressure cell allows the observation of individual protein hydrogen bonds in the NMR instrument under pressures of up to 2500 bar. The latter is equivalent to the hydrostatic pressure of a water column of 25 km height. Hydrogen bonds spanning small sequence separations between the interacting amino acids were found to be particularly stable, whereas hydrogen bonds that span over larger sequence separations showed generally lower stability. Surprisingly, however, there are exceptions to this rule: hydrogen bonds that connect very important parts of Ubiquitin, can span over large sequence separations and be nevertheless extremely stable. In particular, such unusually stable long-range hydrogen bonds were found in the structural part where Ubiquitin attaches to target proteins. By this covalent attachment, ubiquitin labels misfolded target proteins for degradation and fulfills its function in cellular protein quality control. The specific stabilization of hydrogen bonds at this site is therefore very important to preserve the structural integrity of Ubiquitin during function and to achieve stability for the entire protein.
Future oriented technology: High pressure-NMR
By the high pressure NMR characterization, Nisius and Grzesiek could identify the structural parts of Ubiquitin that are responsible for its unusually high thermodynamic stability. Their study is a further example of the multifaceted and growing range of NMR applications. The technology not only provides information on the three-dimensional structure of biomolecules, but also on their thermodynamic and kinetic characteristics, and thus is a crucial tool to understand biomolecular function at atomic resolution.
Further Information:
Lydia Nisius and Stephan Grzesiek:
Key stabilizing elements of protein structure identified through pressure and temperature perturbation of its hydrogen bond network.
In: Nature Chemistry; published online 08 July 2012, DOI 10.1038/nchem.1396
Source: University of Basel, Switzerland
Last update: 10.07.2012
Perma link: https://www.internetchemistry.com/news/2012/jul12/high-pressure-nmr.php
More chemistry: index | chemicals | lab equipment | job vacancies | sitemap
Internetchemistry: home | about | contact | imprint | privacy
© 1996 - 2023 Internetchemistry
