 |
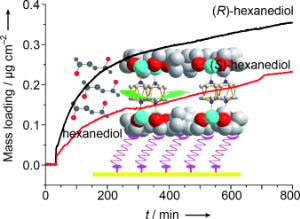
Stuck on you: Preferred (110) and (001) orientation of enantiopure [{Zn2((+)cam)2(dabco)}n] ((+)cam=(1R,3S)-(+)-camphoric acid, dabco=1,4-diazabicyclo(2.2.2)octane) thin films can be controlled by carboxylate and pyridyl groups on self-assembled monolayers (SAMs). With a quartz crystal microbalance, the enantioselective adsorption of enantiomeric hexanediol pairs by enantiopure surface-attached metal–organic frameworks (SURMOF) [Znn2((±)cam)n2(dabco)] pairs can be demonstrated.
[Credit: Angewandte Chemie International Edition] |
 |
This is a molecular framework anchored to a surface separates the enantiomers desired.
[Graphics: KIT-IFG] |
In contrast to conventional methods, the process developed by the team of researchers directed by Professor Wöll, Professor Roland Fischer from the Chair for Inorganic Chemistry II of RUB, and Humboldt scholar Bo Liu (KIT and RUB) allows for a more rapid and, hence, cheaper separation of enantiomers. It is based on novel molecular frameworks (MOFs) that can be grown on solid substrates. These porous coatings that are also referred to as SURMOFs are produced by an epitaxy process specifically developed by the researchers. Instead of heating the solution mixtures produced from the initial substances, modified substrates are immersed alternately in the solutions of the initial substances. "In this way, the molecular layers are assembled one after the other comparable to a rack system," explains Roland Fischer. These molecular rack systems anchored to the surfaces can be functionalized for various applications.
The enantiomers are separated by chiral organic molecules that are the linkers or struts of the rack systems. Thanks to their enantiopure structure, these coatings retain one of both enantiomers. In their contribution that was also selected for the title photo of the journal Angewandte Chemie, the scientists describe the separation of the enantiomer molecules (2R, 5R)-2,5-hexanediol (R-HDO) and (2S, 5S)-2,5-hexanediol (S-HDO). Future work will be aimed at increasing the mesh width of the porous structures in order to test the method for larger molecules used as pharmaceuticals. "Pharmaceutical substances are two or more nanometers in size and, hence, larger than hexanediol. The development of surface-attached networks with such large structures is a big challenge," explains Professor Wöll.
It is a particular advantage of SURMOFs that the efficiency of enantiomer separation can be measured rapidly and precisely. With the help of quartz crystal microbalances, it was demonstrated that surface-anchored molecular framework structures reach excellent separation efficiencies already. "The SURMOFs as a new material have an enormous potential for use in pharmaceutical industry," explains Professor Jürgen Hubbuch, holder of the Chair for Molecular Separation Engineering (MAB) and Spokesman of the KIT Competence Field of Biotechnology.



