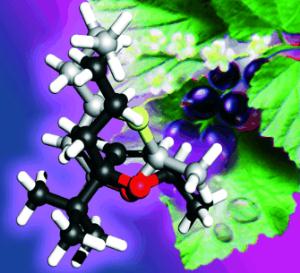
 |
By a combination of microwave spectroscopy and quantum chemistry, the gas-phase structures of the Cassyrane stereoisomers and its dihydro derivatives have been determined, and correlated with their olfactory properties. Superposition analyses of the structures (see picture; black Cassyrane, silver Oxane, red O, yellow S) reveal the importance of the 5S configuration on the cassis odor, with the 2S,5R isomers being the most intense.
[Credit: Angewandte Chemie] |
In addition to their two classic scents, ”Cassis Base 345B” and ”Corps Cassis”, in April 2010 Givaudan introduced a new captive ingredient Cassyrane; this substance imparts a natural, juicy cassis odor with aspects of cassis sorbet upon the top note of a perfume. Cassyrane consists of different so-called isomeric molecules that are of identical atomic composition, but have different spatial arrangements.
When four different atoms are bound to a carbon atom, there are two different ways for these to be arranged relative to each other in space. These two possible structures are mirror images of each other. Natural substances often have several such chiral centers. In scents, each of the possible combinations, known as stereoisomers, can have a different odor that can also be more or less intense. Cassyrane has two chiral centers, which gives it four possible stereoisomers.
Because the cassis odor of the other cassis scents distinctly depends on the configurations of the molecules, the researchers wanted to investigate the scent properties of the individual Cassyrane stereoisomers. They also examined the stereoisomers of the dihydro derivative, a compound of nearly identical structure that also smells of cassis but is missing the double bond found in the Cassyrane molecule.
It was first necessary to synthesize pure forms of each stereoisomer by means of clever procedures. It turns out that not all of the isomers smell of cassis. In both compounds, an R configuration at carbon number 5 elicits a character reminiscent of Provencal herbs like rosemary, while isomers with the 5S configuration had the fruity odor of cassis. The stereocenter at carbon number 2 has a strong influence on the intensity of the odor.
A molecule is a flexible structure; its atomic groups can twist and bend in various ways relative to each other. The researchers wished to determine which of these spatial structures is preferentially adopted by each of these stereoisomers in the gas phase. They were able to achieve this by examining the molecular rotations by means of microwave spectroscopy and combining these results with quantum chemical calculations. When the calculated structures were overlaid with those of the stereoisomers in the classical scents the result was clear: a very specific configuration does seem to be important for the cassis character of the scents.



