 |
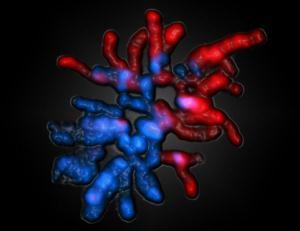
Female and male-derived chromosomes of a fertilized egg of a mouse. The male-derived chromosomes appear red – their genom is already reprogrammated totally. The female-derived chromosomes (blue) are protected against the decoding process.
[Credit: Saarland University, Prof. Walter] |
Jörn Walter, Professor of Epigenetics at Saarland University in Germany whose team led the collaborative work said: “In humans the DNA code is faithfully transmitted from cell to cell and over generations. However, in each new generation the epigenetic code is reset. Epigenetic changes do not alter the genes but affect their interpretation in all cells of a human body. The chromosomes of both parents undergo dramatic and essential epigenetic changes at the beginning of life. Our work deciphers a new chapter in the language and grammar of epigenetic interpretation - the knowledge of this epigenetic language will have a great impact on biomedical research.”
Professor Wolf Reik, Scientist at the Babraham Institute, Cambridge states: “This work provides exciting new insights into how epigenomes are reprogrammed in germ cells and early embryos at the beginning of life. Elucidating these mechanisms may help us to devise better strategies for making stem cell therapies a reality. Also, erasing old epigenetic marks that are inherited from parents and grandparents may be important for healthy ageing and for preventing common diseases such as diabetes or heart disease.”
Epigenetic modification of DNA, for example by methylation of cytosines, enables genes to be switched on or off at different times and places. The reprogramming or ‘erasure’ of such epigenetic tags after fertilisation is essential if cells of a developing embryo are to have the potential to become any type of tissue - a characteristic known as totipotency.
Shortly after fertilisation, the egg starts the unpacking and epigenetic decoding of the sperm’s chromosomes. This maternal “dominance” over the male-derived chromosomes was discovered ten years ago by the authors. A major early event governing the reprogramming is the loss of an epigenetic chemical tag called 5-methylcytosine (5mC). However, there is a striking difference between the demethylation of maternally and paternally-derived chromosomes in the fertilised eggs; the maternal genome appears to protect itself and resist demethylation.
The team discovered that the appearance of 5-hydroxymethylcytosine (5hmC) has a critical role in the process and is associated with structural reorganisation in the nucleus after fertilisation. Within the first hours of development, a strong accumulation of the novel modification 5hmC was seen, almost exclusively on male chromosomes. The egg produces an enzyme called Tet3, which drives the conversion of 5mC into 5hmC. The authors showed that removing this enzyme in the fertilised egg using RNAi methods dramatically decreased the conversion of 5mC into 5hmC. These findings also link the loss of 5mC, first observed by the authors 10 years ago, to its conversion to 5hmC. The authors also discovered a ‘protection factor’ a protein called PGC7, which guards maternal chromosomes against the modification, while allowing “decoding” the paternal DNA-methylation tags by modification into 5hmC. Without PGC7, the methyl groups on maternal chromosomes become accessible for hydroxylation.
While the precise biological role of the large-scale conversion of 5mC into 5hmC remains unclear, the insights are likely to have wide implications for our general understanding of epigenetic reprogramming. It is known that external factors in the environment, or for example in our diet, may have consequences later in life or on future generations. Epigenetics is now established as the ‘integrator’ between the environment and the genome so understanding how epigenomes are modified is key to understanding the mechanisms underpinning lifelong health.
The work was supported by a grant from the Deutsche Forschungsgemeinschaft DFG, an EMBO long-term Fellowship and by the EPIGENOME Network of Excellence.
Epigenetic mechanisms are at the heart of developmental biology, orchestrating the formation of many different tissues and organs from a fertilised egg. Almost all cells in an individual have exactly the same genetic material, yet behave very differently depending on which organs they comprise. It is 25 years since scientists first suspected that there might be heritable biological information separate from the DNA sequence – another inheritance ‘code’. Epigenetic regulation enables the fine tuning of our genes and their expression in different places at different times, leading to the amazing complexity we see in humans despite the relatively small number of unique genes.



