 |
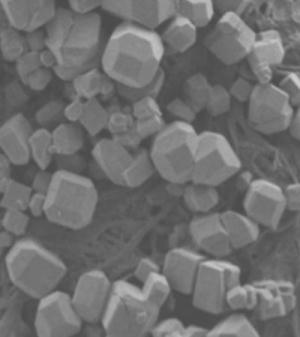
This is a scanning electron microscope image of a new material that self-assembles into a polyhedron using the attractive interactions associated with hydrogen bonds. The shapes then further organize into a crystal lattice that resembles a porous structure called zeolite, an absorbent material with many industrial uses.
[Credit: Michael D. Ward, New York University] |
"We wanted to create frameworks to serve as the 'hotel' for 'guest' molecules, which can deliver the function independent of framework design," said Ward. "This makes it possible to separate chemicals based on size or perform reactions inside well-defined cages, which could potentially give you more control over chemical reactivity and reaction products. Moreover, these frameworks may prove ideal for encapsulating a wide range of guest molecules, producing materials with new optical or magnetic properties."
The molecular "hotels" described by Ward and his collaborators take the shape of a truncated octahedron, one of 13 shapes described as an Archimedean solid, discovered by the Greek mathematician Archimedes. Archimedean solids are characterized by a specific number of sides that meet at corners which are all identical. The regularity of these shapes often means they are of particular interest to chemists and materials researchers looking to create complex materials that assemble themselves.
The extraordinary aspect of this work, supported by the National Science Foundation (NSF), is the self-assembly of the molecular tiles into a polyhedron, a well-defined, three-dimensional, geometric solid. The individual polyhedra assemble themselves using the attractive interactions associated with hydrogen bonds. They then further organize into a crystal lattice that resembles a porous structure called zeolite, an absorbent material with many industrial uses.
The new material differs from zeolite because it is constructed from organic building blocks rather than inorganic ones, which make it more versatile and easier to engineer. In general, inorganic compounds are considered mineral in origin, while organic compounds are considered biological in origin.
This discovery paves the way towards development of a new class of solids with properties that may prove useful for a range of industrial and consumer products.
"By using geometric design principles and very simple chemical precursors, the Ward group has been able to construct relatively sturdy materials which contain many identically sized and shaped cavities," explained Michael Scott, program director in the Division of Materials Research at NSF. "The hollow space inside these materials offers many exciting opportunities for chemists to do things such as isolate unstable molecules, catalyze unknown reactions and separate important chemical compounds."
Future research projects will try to create other types of Archimedean solids or use the truncated octahedron to house different types of functional molecules.
New York University Press Release
Chemists create molecular polyhedron ... and potential to enhance industrial and consumer products
Chemists have created a molecular polyhedron, a ground-breaking assembly that has the potential to impact a range of industrial and consumer products, including magnetic and optical materials.
The work, reported in the latest issue of the journal Science, was conducted by researchers at New York University's Department of Chemistry and its Molecular Design Institute and the University of Milan's Department of Materials Science.
Researchers have sought to coerce molecules to form regular polyhedra-three-dimensional objects in which each side, or face, is a polygon - but without sustained success. Archimedean solids, discovered by the ancient Greek mathematician Archimedes, have attracted considerable attention in this regard. These 13 solids are those in which each face is a regular polygon and in which around every vertex - the corner at which its geometric shapes meet - the same polygons appear in the same sequences. For instance, in a truncated tetrahedron, the pattern forming at every vertex is hexagon-hexagon-triangle. The synthesis of such structures from molecules is an intellectual challenge.
The work by the NYU and University of Milan chemists forms a quasi-truncated octahedron, which also constitutes one of the 13 Archimedean solids. Moreover, as a polyhedron, the structure has the potential to serve as a cage-like framework to trap other molecular species, which can jointly serve as building blocks for new and enhanced materials.
"We've demonstrated how to coerce molecules to assemble into a polyhedron by design," explained Michael Ward, chair of NYU's Department of Chemistry and one of the study's co-authors. "The next step will be to expand on the work by making other polyhedra using similar design principles, which can lead to new materials with unusual properties."
The research team's creation relies on a remarkably high number of hydrogen bonds - 72 - to assemble two kinds of hexagonal molecular tiles, four each, into a truncated octahedron, which consists of eight molecular tiles. Although chemists often use hydrogen bonds because of their versatility in building complex structures, these bonds are weaker than those holding atoms together within the molecules themselves, which often makes larger scale structures constructed with hydrogen bonds less predictable and less sustainable. The truncated octahedron discovered by the NYU team proved to be remarkably stable, however, because the hydrogen bonds are stabilized by the ionic nature of the molecules and because no other outcomes are possible. In fact, the truncated octahedra assemble further into crystals that have nanoscale pores, resembling a class of well-known compounds called zeolites, which are made from inorganic components.
Because the structure also serves as a molecular cage, it can house, or encapsulate, other molecular components, giving future chemists a vehicle for developing a range of new compounds.



