Sooner or later, a cut to the skin or a broken bone will heal on its own; however, a scratch to a car"s paint or a tear in the wing of an airplane will not.
Materials with self-healing properties could help extend the durability of products and make repairs easier.
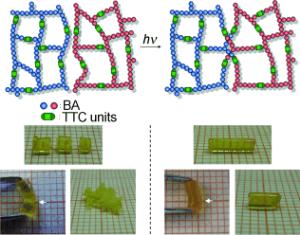
Krzysztof Matyjaszewski and his co-workers at Carnegie Mellon University (Pittsburgh, USA) and Kyushu University (Japan) have now developed a polymer that can repair itself when irradiated with UV light - over and over again. As the scientists report in the journal Angewandte Chemie, this is the first material in which capped covalent bonds repeatedly reattach, even allowing fully separated pieces to be fused back together.
Some previous solid self-healing materials contain tiny capsules that tear open to release a chemical agent when the material is damaged and have been able to repair themselves only one time. Other materials, including some gels, can repair themselves repeatedly but lack the covalent bonds that increase materials strength and stability.
In contrast, the new polymeric material produced by the American and Japanese team is stable and repairs itself again and again. The secret to their success is that the polymer is cross-linked through trithiocarbonate units. These are carbon atoms bonded to three sulfur atoms, two of which use their second bonding position to attach to another carbon atom. These groups have a special property: they can restructure under UV light. The light breaks one carbon - sulfur bond in the trithiocarbonate groups. This produces two radicals - molecules with a free, unpaired electron. The radicals are very reactive and attack other trithiocarbonate groups to form new carbon-sulfur bonds while breaking others to form more free radicals. The chain reaction stops when two radicals react with each other.
The researchers were able to heal cut polymer fragments with irradiation - either immersed in liquid or in bulk. They only had to firmly press the cut edges together and irradiate them. The edges grew back together by means of the radical re-organization process described above.
The self-healing effect goes much further: even shredded polymer samples could simply be pressed together and irradiated to be fused into a continuous piece. The resulting object was in the shape of the cylindrical tube in which the procedure was carried out. This self-healing process can be carried out repeatedly on the same sample. The material is thus also interesting as a new recyclable product.
Further Information:
Yoshifumi Amamoto, Jun Kamada, Prof. Hideyuki Otsuka, Prof. Atsushi Takahara, Prof. Krzysztof Matyjaszewski:
Repeatable Photoinduced Self-Healing of Covalently Cross-Linked Polymers through Reshuffling of Trithiocarbonate Units..
In: Angewandte Chemie International Edition; article first published online: 10 January 2011, DOI 10.1002/anie.201003888
Source: Angewandte Chemie International Edition, press release 49/2010
Last update: 19.01.2011.
Perma link: https://www.internetchemistry.com/news/2011/jan11/self-healing-materials.php
© 1996 - 2023 Internetchemistry