 |
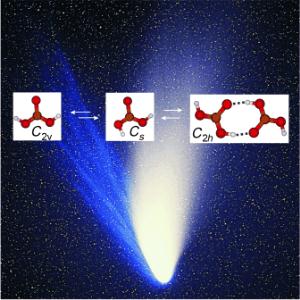
Against all odds: Carbonic acid molecules were trapped from the gas phase in a solid noble-gas matrix at less than 10 K and studied by IR spectroscopy. The 2H and and 13C isotopologues were also examined. Gas-phase carbonic acid is thought to exist as a 1:10:1 mixture of two monomeric conformers and the cyclic dimer (H2CO3)2. This data is vital in the search for gas-phase carbonic acid in astrophysical environments.
[Source: Angewandte Chemie, Wiley-VCH] |
Carbonated beverages contain carbon dioxide. They also contain trace amounts of a molecule that was long thought to be too unstable to exist: carbonic acid (H2CO3). It is now known that carbonic acid is indeed present in drinks, though at very, very low concentrations. Until recently, the molecule has resisted all attempts at isolation and direct detection. However, a few scientists have been able to produce carbonic acid in the solid state. It is also assumed to be present in cirrus clouds in Earth’s atmosphere and in space.
The Austrian researchers have now demonstrated that carbonic acid can exist in the gas phase and that it is stable at temperatures up to –30 °C. For these experiments, solid carbonic acid was formed by means of acid-base reactions at very low temperatures and then warmed to –30 °C. The evaporating molecules were trapped in a matrix of the noble gas argon and then immediately cooled again. This resulted in a kind of frozen “image” of the gas-phase carbonic acid, which the researchers were able to study by infrared spectrometry.
The spectra showed that gas-phase carbonic acid exists in three different forms. The scientists found two monomers that differ in their conformation - the spatial arrangement of their atoms - as well as a dimer made from two molecules bound through hydrogen bonds.
The resulting detailed spectrometric data are of great interest to astronomers, because they could make it easier to detect gas-phase carbonic acid in space, where it is thought to be present in the tails of comets and on Mars.
Press release: University of Innsabruck, Austria:
Gas-phase Carbonic Acid Isolated
A team of chemists headed by Thomas Loerting
from the University of Innsbruck and Hinrich
Grothe from the Vienna University of Technology
(TU Wien) in Austria have prepared and isolated
gas-phase carbonic acid and have succeeded in
characterizing the gas-phase molecules by using
infrared spectroscopy. The results were
published in the journal Angewandte Chemie
International Edition.
In textbooks and other media the widespread
belief still prevails that stable carbonic acid
cannot be produced in pure form and is
practically non-existent as it immediately
decomposes to carbon dioxide and water. However,
Innsbruck chemists headed by Erwin Mayer
(Institute of General, Inorganic and Theoretical
Chemistry) refuted this persistent dogma in
chemistry several years ago. They belong to only
a handful of scientists who have prepared pure
solid carbonic acid experimentally. In an
international first, the scientists have now
produced gas-phase carbonic acid and, together
with a research group headed by Hinrich Grothe
at the Vienna University of Technology, they
have also succeeded in proofing the existence of
these molecules. “Carbonic acid vapor is
composed of at least three different species in
the gas-phase: a cyclic dimer consisting of two
molecules and two different types of monomers,“
explains Thomas Loerting (Institute of Physical
Chemistry) the result of the comprehensive
study.
Surprising result
For this experiment the researchers prepared
carbonic acid in the laboratory in Innsbruck. It
was then stored in liquid nitrogen and
transported to Vienna by PhD student Jürgen
Bernard. At the Institute of Materials Chemistry
at the TU Wien the solid carbonic acid was
warmed to minus 30 degrees Celsius. “During this
process the carbonic acid molecules entered the
gas-phase,“ says Loerting. This is a surprising
result because many experts in the field
believed that carbonic acid immediately
decomposes to carbon dioxide and water. The
Austrian scientists trapped the carbonic acid
vapor in a solid matrix of the inert gas argon
and cooled it down. “This produced a frozen
image of the carbonic acid vapor, which we
analyzed by using high-resolution infrared
spectroscopy at the TU Wien,“ says Hinrich
Grothe. “The spectrum we produced is extremely
precise and we were able to assign the spectral
bands to the vibration of each single molecule.“
For more than a decade, the chemists have been
supported in their experimental research by
Klaus Liedl from the Institute of Theoretical
Chemistry in Innsbruck. His team of scientists
has helped to interpret the experimental data
with computational models. Additional
calculations have been performed by Oscar Galvez
from CSIC Madrid (Spanish National Research
Council).
Infrared spectra in research
This experiment not only is of high importance
for basic research but also for astronomy. The
identification of gas-phase carbonic acid in the
atmosphere of celestial bodies may be
facilitated by the detailed spectra of gas-phase
carbonic acid described in this study.
“Conditions in space environments suggest that
gas-phase carbonic acid may be found in the coma
of comets or the poles of Mars,“ says Thomas
Loerting. “However, infrared spectra currently
measured in extraterrestrial environments are
still too imprecise to be comparable to the
results produced in our laboratory.“
The team of chemists headed by Loerting and
Liedl are members of the research platform
Advanced Materials of the University of
Innsbruck and are supported by the Austrian
Science Fund (FWF) and the European Research
Council (ERC). The chemists in Vienna supervised
by H. Grothe participate in the TU Wien
Materials Research Cluster and are supported by
the Austrian Exchange Service (ÖAD).



