|
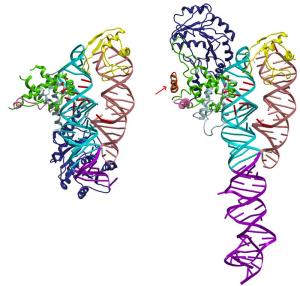
Structure of SRP without its address label, that is, the signal sequence, (above) and with the signal sequence (right). The signal sequence is marked with a red arrow.
[Credit: Swedish Research Council] |
The capacity to transport proteins in most cases is directly linked to the function of the SRP, the signal-recognizing particle. The SRP binds to the signal sequence and guides it and the attached protein to the cell membrane. A key question for these researchers has been how the interaction between the signal sequence and SRP works in detail.
The Umeå scientists have managed to create a detailed picture of the first step in this protein transport by studying a complex of a signal sequence that is bound to the SRP. The technology they used is called x-ray crystallography. The group has shown the basic structure of the SRP in several previous studies SRP. Thanks to these studies, they were now able to directly compare the SRP structure with and without the guiding signal sequence.
”The structural changes were considerably greater than what was previously predicted. They provide us with detailed explanations of what role SRPs play in protein transport. These structural specifications can also serve as a model of how SRPs function at various levels during protein transport," explains Elisabeth Sauer-Eriksson, professor at the Department of Chemistry.
Now these researchers are moving on to try to investigate the next transport mechanism. For instance, they want to answer questions about what prompts the bound signal sequence to let go of the SRP and how the signal sequence, and the protein it is attached to, can make its way through the membrane.
The scientists who carried out the study are part of Umeå University's strong research environment "biological chemistry" and the Umeå Centre for Microbial Research, UCMR. Funding for the research project is provided by the Swedish Research Council, UCMR, and the Kempe Foundations.
About SRP
SRP is a ribonucleotide protein complex. SRP is highly conserved in nature and exists in all living organisms, which indicates that it plays a fundamental role in the structure and function of the cell.



