 |
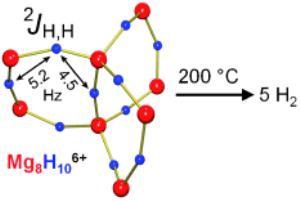
Big can be small: The largest ligand-stabilized magnesium hydride cluster, containing 8 Mg2+ and 10 H- ions, is a model for the smallest sub-nanometer-sized hydrogen storage material. This molecular cluster displays magnetic hydride–hydride coupling (see picture) and complete hydrogen desorption at the record-low temperature of 200?°C.
[Credit: Angewandte Chemie] |
Magnesium hydride (MgH2) can release hydrogen when needed and the resulting magnesium metal reacts back again to form the hydride by pressurizing with hydrogen at a "gas station". Unfortunately, this is an idealized picture. Not only is the speed of hydrogen release/uptake excessively slow (kinetics) but it also only operates at higher temperatures (thermodynamics). The hydrides, the negatively charged hydrogen atoms (H-), are bound so strongly in the crystal lattice of magnesium cations (Mg2+) that temperatures of more than 300 °C are needed to release the hydrogen gas.
Particularly intensive milling has made it possible to obtain nanocrystalline materials, which, on account of its larger surface, rapidly release or take up hydrogen. However, the high stability of the magnesium hydride still translates to rather high release temperatures. According to recent computer calculations, magnesium hydride clusters of only a few atoms possibly could generate hydrogen at temperatures far below 300 °C. Clusters with less than 20 Mg2+ ions are smaller than one nanometer and behave differently from the bulk material. Their hydride ions have fewer Mg2+ neighbors and are more weakly bound. However, it is extremely difficult to obtain such tiny clusters by milling. In Harder's "bottom-up" approach, magnesium hydride clusters are made by starting from molecules. The challenge is to prevent such clusters from forming very stable bulk material. Using a special ligand system, they could trap a cluster that resembles a paddle wheel made of eight Mg2+ and ten H- ions. For the first time it was shown that molecular clusters indeed release hydrogen already at the temperature of 200 °C.
This largest magnesium hydride cluster reported to date is not practical for efficient hydrogen storage but shines new light on a current problem. It is easily studied by molecular methods and as a model system could provide detailed insights in hydrogen storage.



