The protein Hsp90 plays a significant role in the survival of cells that are exposed to stress. Researchers at the Technische Universitaet Muenchen (TUM) uncovered this protein's mode of operation some time ago - but now Hsp90 has surprised even the experts with an unexpected pattern of motion. The results are published in the online issue of the renowned science journal PNAS and may help researchers discover specific cancer medication.
Proteins are the motors of the cell: They transport, among other things, nutrients, move our muscles, convert substances chemically or fold other proteins. The so-called heat shock protein Hsp90 is eminently important for our cells since it plays a decisive role in many basic processes - in humans as well as in bacteria or yeasts. For example, it is decisive in folding polypeptide chains into functioning proteins with very precisely defined spatial structures. Especially when cells are exposed to stress through heat or poisonous substances, Hsp90 production increases to keep the damage in check.
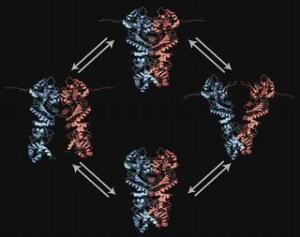
The anti-stress protein is a dimer (which consists of two identical proteins) and can be roughly divided into three segments: the N terminal domain at the top, the middle domain and the C terminal at the bottom. Hsp90 taps the energy it requires for its work from the slow splitting of ATP, the fuel of every cell. In this process, the two strands move in opposing directions, albeit only a few nanometers. The TUM groups of Prof. Thorsten Hugel from the Department of Physics and Prof. Johannes Buchner from the Department of Chemistry are quite familiar with the movement of Hsp90: They were the first to observe the scissor-like movement in real-time. Yet even they were caught off guard: Instead of the familiar one-ended scissor movement at the N terminal domains they were now observing the rocking movement at both ends of the protein.
Hsp90 opens and closes in a scissors-like manner at the C terminal as well - something hitherto unknown in dimers. The researchers relied on the so-called FRET technology (FRET = Förster Resonance Energy Transfer) in their new study. They attached two fluorescent molecules at precisely defined positions in the Hsp90 and used these as a molecular ruler: When one pigment is illuminated, the other glows with increasing intensity the closer the two pigments get to each other. Using this effect, they were able to observe the nanometer-scale, double-ended scissor movement in individual Hsp90 dimers using a specially constructed microscope which allowed to study single molecules.
Particularly interesting is that the double scissor movements at the N and C terminals are closely coupled: The Hsp90 dimer obviously opens and closes in alternation at each end, like a rocker. "That explains the great stability of the dimer - otherwise Hsp90 would fall apart much faster," explains Thorsten Hugel. His team was also very surprised by the mechanism regulating the speed of the rocking motion: it is the ATP bound at the N terminal domains that regulates the motion at the C terminal end. The researchers showed this by switching off the dimer's ATP energy supply. The team concluded that Hsp90 communicates internally across an unusually long distance of almost ten nanometers.
The observed movement and communication patterns are interesting not only for basic research, but also for medical research since Hsp90 is a new drug target in cancer therapy. The most promising drug candidates to date block the binding of ATP at the N terminal domains of the anti-stress protein. However, these compounds may have undesirable side effects. Thanks to their new insights, the TUM researchers can now concentrate on the C terminal dimerisation of Hsp90: "There we have unique docking points for anti-cancer drugs that should function without side effects," says Hugel.
The research was funded by the Deutsche Forschungsgemeinschaft (German Research Foundation), the Fonds der Chemischen Industrie (Chemical Industry Fund) and the two Excellence Clusters Nanoinitiative München (NIM) and Munich Center for Integrated Protein Science (CIPSM).
Further Information:
C. Ratzke, M. Mickler, B. Hellenkamp, J. Buchner, and T. Hugel:
Dynamics of heat shock protein 90 C-terminal dimerization is an important part of its conformational cycle.
In: Proceedings of the National Academy of Sciences; published online before print August 24, 2010, DOI 10.1073/pnas.1000916107
Source: Technical University of Munich, Germany. TUM
Last update: 01.09.2010
Perma link: https://www.internetchemistry.com/news/2010/sep10/hsp90-protein-rocking-movement.php
More chemistry: index | chemicals | lab equipment | job vacancies | sitemap
Internetchemistry: home | about | contact | imprint | privacy
© 1996 - 2023 Internetchemistry
