Carbon monoxide is an insidious poison: it is colorless, odorless, and toxic at low concentrations. It is usually produced by combustion engines or incomplete combustion in gas furnaces or wood-burning fireplaces.
Spanish researchers working with Ramón Martínez-Máñez have now developed a sensitive and selective detector that reliably detects CO in air. As the scientists from the IDM Research Institute at the Polytechnic University of Valencia report in the journal Angewandte Chemie, their system involves a special rhodium complex that distinctly changes color in the presence of CO.
In the USA alone, there are 15,000 accidents with CO reported annually; 500 of these are fatal. The implementation of reliable warning devices in dangerous locations is thus correspondingly important. Most modern CO sensors are electronic; as an alternative, researchers are looking for detectors that indicate the presence of CO by a color change. However, such colorimetric detection methods remain rare, don"t function in air, or are not sensitive enough.
Based on a special complex of rhodium, the Spanish researchers have now developed a CO detector that not only reliably detects CO in solution, but also in air. The detection limit is low enough that it responds before toxic levels are reached.
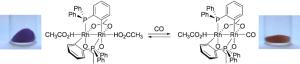
At the core of the complex are two rhodium atoms connected to each other by acetate groups and two special phosphorus-containing ligands (cyclometallated phosphines). The metals are also bound axially by two acetic acid ligands. The complex molecule is deposited onto silica gel, where it is adsorbed; this forms a gray-violet solid. If the complex comes into contact with air containing CO, one or two molecules of CO bind to the rhodium by forcing the acetic acid molecules out of their axial binding sites on the two rhodium atoms. Within a few minutes, this causes a distinct color change in the solid, which becomes orange-yellow. Treatment with a stream of clean air completely regenerates the detector.
The detection system is highly selective for CO. It does not respond to CO2, volatile organic compounds, or SO2. It only reacts to NO2 when it is present in extremely high concentrations. The researchers hope that this system will form the basis for efficient, low-maintenance chemosensors for the easy and inexpensive detection of CO. "For instance colorimetric detection systems of CO can be implemented in clothes, paintings etc.," says Martínez-Máñez, "and the presence of CO will then simply be detected via a color change visible to the naked eye". In contrast, electronic equipment needs a source of electricity and is difficult to incorporate into the fabric of clothes.
Further Information:
Dr. Julio Esteban, Dr. José Vicente Ros-Lis, Prof. Ramón Martínez-Máñez, Dr. M. Dolores Marcos, María Moragues, Dr. Juan Soto, Dr. Félix Sancenón:
Sensitive and Selective Chromogenic Sensing of Carbon Monoxide by Using Binuclear Rhodium Complexes.
In: Angewandte Chemie International Edition; Volume 49 Issue 29, Pages 4934 - 4937, published online: 2 Jun 2010, DOI 10.1002/anie.201001344
Source: Angewandte Chemie International Edition, press release 23/2010
Last update: 07.07.2010
Perma link: https://www.internetchemistry.com/news/2010/jul10/carbon-monoxide-detection-with-a-rhodium-complex.php
More chemistry: index | chemicals | lab equipment | job vacancies | sitemap
Internetchemistry: home | about | contact | imprint | privacy
© 1996 - 2023 Internetchemistry
