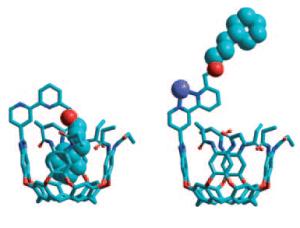
Image below: A molecular switch - The ouroborand coordinates an internal side chain in its cavity, just as it were swallowing its own tail. The presence or absence of zinc(II) in solution switches the cavity between open and closed states to external guests (see scheme: deep blue sphere: Zn). [Credit: Angewandte Chemie International Edition, Wiley-VCH]
The ouroboros (ancient Greek for "tail devourer") is a motif found in many cultures: a snake biting its own tail, it symbolizes eternity and cycles.
Julius Rebek, Jr. and Fabien Durola (The Scripps Research Institute, La Jolla, USA) have now constructed a molecular tail devourer, and have named this new class of compound "ouroborand".
As the researchers report in the journal Angewandte Chemie, their tail-biter is a molecular "machine", which functions as a nanocontainer with a built-in switch that regulates access to the cavity.
Molecular machines and nanoscopic components imitate - at least theoretically - the functions of their macroscopic analogues. For example, nanoscopic capsules can act as reaction vessels, molecules with parts that rotate relative to each other to imitate rotors, and various types of on/off switches.
The ouroborand made by the American research duo is a molecule consisting of multiple parts. A cavity that can take up guest molecules serves as a container. At its edge, the container has a switchable rotor (a bipyridyl unit) to which an intramolecular guest is attached like a hand at the end of a coupling arm of appropriate length. The rotor is turned so that the hand at the end of the arm sits inside the container. The container is thus blocked and not accessible to other molecules; it is switched to closed. In this conformation it is reminiscent of a snake that is swallowing its own tail, the ouroboros.
If zinc ions are added to the solution, they trigger a switching mechanism: the rotor has two binding sites for zinc ions. In order for both to bind an ion, the rotor must make a half-turn. The coupling arm turns with it, which causes the hand to be pulled out of the container. The vessel is now free and accessible to other molecules; it is switched to open. If the zinc ions are taken back out of the solution, the rotor then turns back to the starting position and the hand throws the foreign molecule back out of the container.
Further Information:
Dr. Fabien Durola, Prof. Dr. Julius Rebek Jr.:
The Ouroborand: A Cavitand with a Coordination-Driven Switching Device.
In: Angewandte Chemie International Edition; Volume 49 Issue 18, Pages 3189 - 3191, 18 Mar 2010, DOI 10.1002/anie.200906753
Source: Angewandte Chemie International Edition, press release 14/2010
Last update: 30.04.2010
Perma link: https://www.internetchemistry.com/news/2010/apr10/ouroborands.php
More chemistry: index | chemicals | lab equipment | job vacancies | sitemap
Internetchemistry: home | about | contact | imprint | privacy
© 1996 - 2023 Internetchemistry
