When the human body becomes infected with new influenza viruses, the immune system rapidly activates an inborn protective mechanism to inhibit the intruding pathogen. A protein known as Mx plays an important role in this process, keeping the spread of viruses in check. Exactly how Mx accomplishes this task was previously unknown.
Now virologists from the Institute of Medical Microbiology at the Freiburg University Medical Center and structural biologists from the Max Delbrück Center for Molecular Medicine (MDC) in Berlin-Buch, Germany, have unraveled the structure of the Mx protein and are able to explain how it develops its anti-viral effect.
New influenza viruses jump from animals to humans with alarming frequency, as evidenced by the H5N1 bird flu virus or, more recently, with the swine flu virus. Although humans usually do not have any preexisting immunity to such pathogens, they are not completely unprotected against the invaders. The human body can rapidly mobilize a defense strategy which prevents the influenza viruses from proliferating unchecked in the body.
An essential element of this protection is a protein, known as Mx (short for myxovirus resistance), produced by the body which recognizes many viruses and prevents them from replicating inside infected cells. Under normal conditions this protective protein is not present in the cell at all, but after infection it can be produced in large quantities. The order to produce this protein Mx is made by the signaling protein interferon, which is excreted by infected cells and alarms the organism of the virus infection.
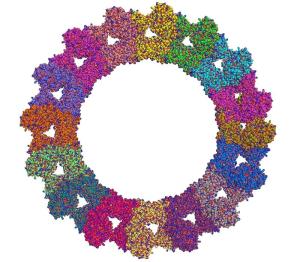
Mx is a molecular machine which does not develop its full power until the individual molecules have joined to form a ring-structured macromolecular network. A central element of the formation of these ring structures is the special part of Mx known as the stalk.
Scientists have attempted to describe the structure of this stalk for years. The virologists Otto Haller, Alexander von der Malsburg, and Georg Kochs in Freiburg and the structural biologists Oliver Daumke, Song Gao, Susann Paeschke, and Joachim Behlke from MDC in Berlin-Buch have now unraveled the secret of the stalk structure of Mx at the atomic level. This structure explains the composition of Mx and allows scientists to conduct tests to make predictions concerning the mode of action of the antiviral molecule.
In combination with findings from earlier biochemical studies, the results of this study make it clear that the stalk structure of Mx functions as a kind of clamp which restrains and deactivates important components of the influenza virus in the infected cell. The fact that new forms of flu can lead to epidemics or even pandemics in spite of this defense mechanism is due to the power and aggressiveness of these pathogens.
The researchers are confident that their new findings about the protective Mx protein will form the basis for the development of new antiviral drugs for combating dangerous influenza viruses. Moreover, they are also certain that this new knowledge about the function of Mx will increase their understanding of other members of this family of proteins.
Further Information:
Song Gao, Alexander von der Malsburg, Susann Paeschke, Joachim Behlke, Otto Haller, Georg Kochs, Oliver Daumke:
Structural basis of oligomerization in the stalk region of dynamin-like MxA.
In: Nature; advance online publication 28 April 2010, DOI 10.1038/nature08972
Source: Max Delbrück Center for Molecular Medicine, MDC, Berlin, Germany
Last update: 30.04.2010
Perma link: https://www.internetchemistry.com/news/2010/apr10/myxovirus-resistance-protein.php
More chemistry: index | chemicals | lab equipment | job vacancies | sitemap
Internetchemistry: home | about | contact | imprint | privacy
© 1996 - 2023 Internetchemistry
